Content – Other fuels
Carbon dioxide (CO2) is a colorless, odorless gas, vital to life on Earth.
Carbon dioxide together with light energy from the sun is absolutely necessary for plants and other organisms to generate oxygene for the atmosphere through the photosynthesis.
Carbon dioxide is composed of one carbon atom bonded to two oxygen atoms.
Carbon dioxide exists in the atmosphere at a concentration, currently of about 0.04 % (400 ppm) by volume.
Natural carbon dioxide sources include volcanoes, hot springs and geysers and it is freed from carbonate rocks by dissolution in water and acids.
Since carbon dioxide is soluble in water, it occurs naturally in groundwater, rivers and lakes, in ice caps and glaciers and in seawater. It is present in deposits of petroleum and natural gas.
Atmospheric carbon dioxide is the primary source of carbon in life on Earth and its concentration in Earth’s pre-industrial atmosphere since late in the Precambrian was regulated by photosynthetic organisms and geological phenomena. As part of the carbon cycle, plants, algae, and cyanobacteria use light energy to photosynthesize carbohydrate from carbon dioxide and water, with oxygen produced as a waste product.
Carbon dioxide exchange and generation:
- By plants during respiration.
- By respiration of all aerobic organisms.
- By decomposition of organic materials.
- By fermentation of sugars
- By combustion of wood, carbohydrates and fossil fuels.
- By Ocean – Atmosphere exchange
Carbon dioxide has a wide area of industrial use:
- Inert gas during welding
- Inert gas in fire extinguishers
- Pressurizing gas (oil recovery, tools, household and leisure appliances)
- Chemical feedstock
- Stimulation of plant growth (green houses)
- Pentacide (green houses)
- Solvent (liquid form)
- Carbonization of beverage
- Refrigrant (frozen solid – “dry ice”)
- Abrasive (frozen solid – “dry ice”)
Plant Respiration
During respiration plants release CO2 back to the atmosphere. Respiration occurs as plant cells use carbohydrates, made during photosynthesis, for energy.
Plant respiration represents 60 Gt/year (around half of the CO2 that is returned to the atmosphere in the terrestrial portion of the carbon cycle).
Soil Respiration
CO2 is realesased into the atmosphere when dead organic matter is broken down or decomposed (consumed by bacteria and fungi). Soil respiration represents 60 Gt/year.
Ocean – Atmosphere exchange
At the interface of the ocean surface and the atmosphere inorganic carbon is absorbed and released through the process of diffusion.
In a dissolved form, CO2 react with H2O to form H2CO3 (carbonic acid, the anion of which, CO3, is called carbonate). The formation of carbonate in seawater allows oceans to take up and store a larger amount of carbon than would be possible if dissolved CO2 remained in that form. Carbonate is also important to a vast number of marine organisms that use this mineral form of carbon to build shells.
Carbon is also cycled through the ocean by the biological processes of photosynthesis, respiration, and decomposition of aquatic plants.
Marine organisms decompose at a much higher speed than on land vegetation. For this reason, very little carbon is stored in the ocean through biological processes. The total amount of carbon uptake, about 92 Gt and carbon loss about 90 Gt from the ocean is dependent on the balance of organic and inorganic processes.
Fossil fuel combustion
Fossil fuels (coal, oil and natural gas) contain carbon that was captured by living organisms over periods of millions of years and has been stored within the Earth crust The main byproducts of fossil fuel combustion is CO2 and H2O, Currently fossil fuel combustion represents a flux of CO2 to the atmosphere of approximately 6-8 Gt/year.
Simplified Example (assuming complete (100%) combustion):
- Combustion of 1 kg gasoline (Octane) (for simplicity the actual sulphur content of the fuel and the nitrogen content of the air is not included):
Balanced reaction:
C8H18 + 12,5 O2→ 9 H2O + 8 CO2 + heat energy
Mass balance:
Molecular weight
C ≈ 12 g/mol
O ≈ 16 g/mol
H ≈ 1 g/mol
1 kg C8H18 needs 3,51 kg O2 to obtain complete combustion.
This generates: 1,4 kg H2O and 3,09 kg CO2
Demonstrated by the mass balance calculation:
\dfrac{8\times12}{114}1000g(C)+\dfrac{18\times1}{114}1000g(H)+\dfrac{12,5\times2\times16}{114}1000g(H)
=9((1\times2)+16)\dfrac{1000}{114}g(H_2O)+8(12+(2\times16))\dfrac{1000}{114}g(CO_2)
Since gasoline has a specific weight of 0,75kg/litre, combustion of 1 litre of gasoline generates: 1,05 kg H2O and 2,32 kg CO2
- Combustion of 1 kg methane:
Balanced reaction
CH4 + 2 O2→ 2 H2O + CO2 + heat energy
By applying the mass balanced calculation
Burning 1 kg of methane generates 2,7 kg of CO2
- Combustion of heavy fuel oil/bunker oil:
2 C20H42+ 61 O2→ 42 H2O + 40 CO2
By applying the mass balanced calculation
Burning 1 kg of heavy fuel oil will generate 3,12 kg of CO2.
- Combustion of diesel
C12H26+ 18,5 O2→ 13 H2O + 12 CO2 + heat energy
By applying the mass balanced cal
Burning 1 kg of diesel oil generates 3,105 kg of CO2.
Deforestation and changes in land use
The main effects that deforestation and change in land use has on the carbon dioxide exchange and emissions are:
- Release of CO2 from disposal of plants and wood (decomposition or by burning).
- Reduced storage of carbon from CO2 absorbation (photosyntesis due to less forest)
- Increased release of CO2 from the soil where forest has been removed.
The net yearly effect of deforesting and change in land use is estimated to an increased CO2 flux of about 1,5 Gt/year.
Annual growth in global carbon dioxide emissions related to primary energy consumption
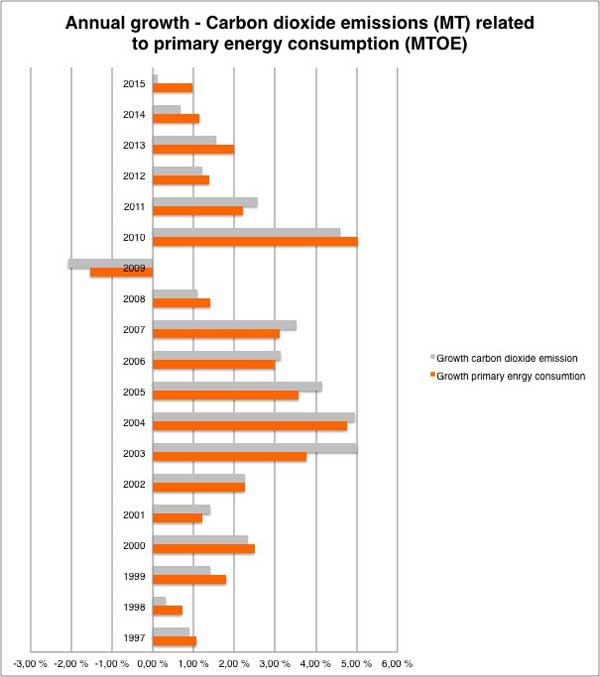
For more data on global carbon dioxide emissions related to energy consumption view our charts: Carbon dioxide emissions