![]() By:Lars Engvik
By:Lars Engvik
It is common knowledge that the use of fossil fuels (hydrocarbons and coal) in combustion engines and in other appliances and machinery where combustion takes place, represents a large amount of the global CO2 emissions as well as other undesired emissions such as soot, NOx and SOx.
It is also obvious that we do need energy to support the growing population of the Earth, to sustain the standard of living in developed countries and to grow the standard of living in developing countries.
Generation of Zero or low-emission energy still represents only a small part of the global energy production.
What can we do?
Instead of “fighting” over the environmental consequences of using oil and gas potentially preventing development of sound solutions and potentially causing a lot of resources to be left in the ground, why not explore the opportunities provided by existing technologies at the same time as we significantly reduce emissions.
This way we may move faster towards a low emission society without going in circles, waiting for the results of comprehensive research programs and time consuming product and process development.
Before I give an example of how we could do this, I would like to remind you that all transition, conversion, refining or use of energy or energy carriers implies “wasting” some of the energy due to thermal losses. We therefore need to look for compromises considering cost, energy efficiency and environmental issues.
How can we do it?
We “simply” split the oil and gas molecules into its basic building blocks, namely hydrogen and carbon. Since we then remove the carbon in solid form there will be no direct CO2 emissions.
Hydrogen has a great potential of becoming a major energy carrier with its energy density of around 33 kWh/kg. When hydrogen reacts with oxygen in a fuel cell, only electricity and water is produced, there are no other emissions such as CO2.
The carbon produced, in the form of Carbon Black is an attractive commercial product.
With the recent developments of fuel cells for fuel cell electrical vehicles and for other electricity generation the demand for pure hydrogen is expected to rise significantly over the coming years.
Carbon Black is a carbon-based powder used in many applications such as additive in rubber, in particular rubber tires, in plastics, in paper, in ink and toner products and many more.
Hydrogen is the most common element in the universe but since hydrogen is not freely available, some form of processing is always necessary to generate hydrogen.
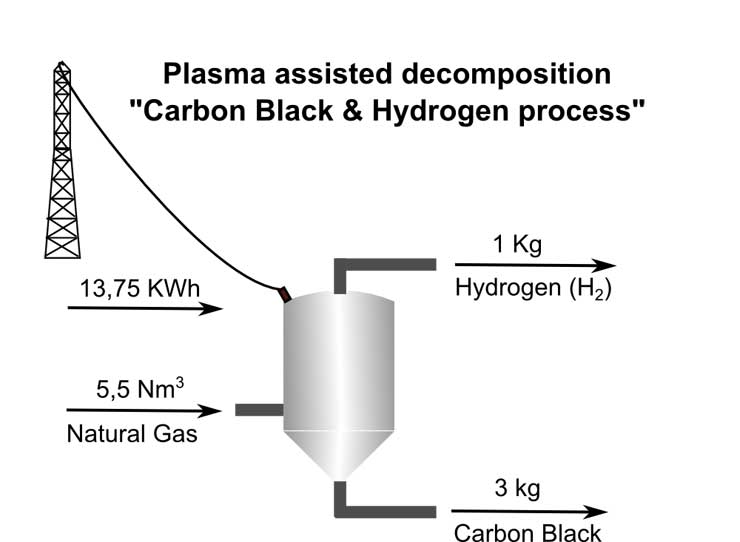
One well-proven method for splitting of the hydrocarbon molecules is Plasma Assisted Decomposition as represented by the Carbon Black & Hydrogen (CB & H) process developed by the company named Kvaerner during the 1990s.
The process includes heating of the feed material to very high temperatures, 1000 °C and more, with no oxygen present. To maximise the energy efficiency it is necessary to regenerate as much as possible of the heat. This is normally obtained by preheating the feed material, the remaining heat is used to produce steam for electricity generation or to cover other heating needs.
Hydrogen and Carbon Black is separated through centrifuges and filters.
Oil and gas are excellent feedstock for hydrogen production due to the high hydrogen content. Methane for example, contains around 33% hydrogen by weight, as compared to water used for electrolysis, which contains around 12,5% hydrogen.
The CB & H process involves pyrolysis of methane or any other hydrocarbon for that sake, with a moderate need for other energy input, around 1 – 1,5 kWh/ Nm3 (kilowatt hour per normal cubic metre) hydrogen produced.
Methane is the feed materiel providing the highest hydrogen yield due to its high hydrogen content.
The beauty of this process is that the emissions are very low, the “well to wheel” emissions, is of course pending on how the electricity is generated and the production method of the hydrocarbons used. The process itself only produces hydrogen and Carbon Black and utilise the feed material close to 100%.
Another method for splitting hydrocarbons into hydrogen and carbon is Thermo Catalytic Decomposition, still not fully commercialised but the method have potential to provide hydrogen and carbon with close to zero emissions since the energy demand could be covered by utilising some of the hydrogen produced for heat generation.
Why do we not do this today?
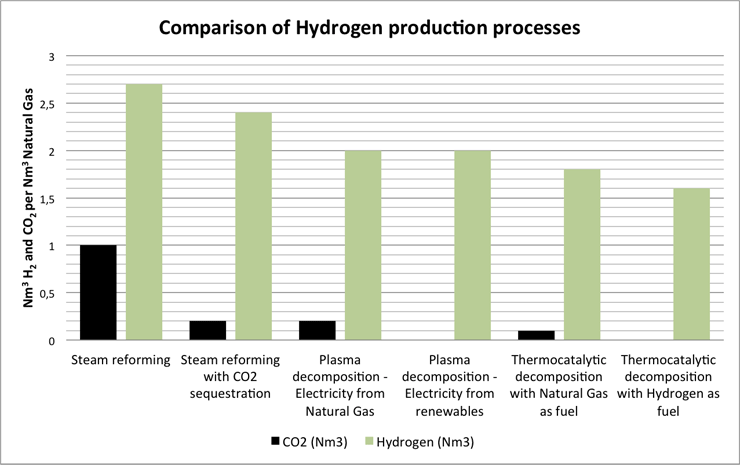
Well, we do but not to a large extent. The main reason being that the current need for hydrogen, which primarily is for technical use within refineries ammonia plants and other industrial applications is produced by cheaper processes emitting large amounts of CO2, such as steam reforming of methane without CO2 capture and storage or it is produced by electrolysis requiring a large amount of electrical energy input (4-5 kWh/Nm3 hydrogen produced).
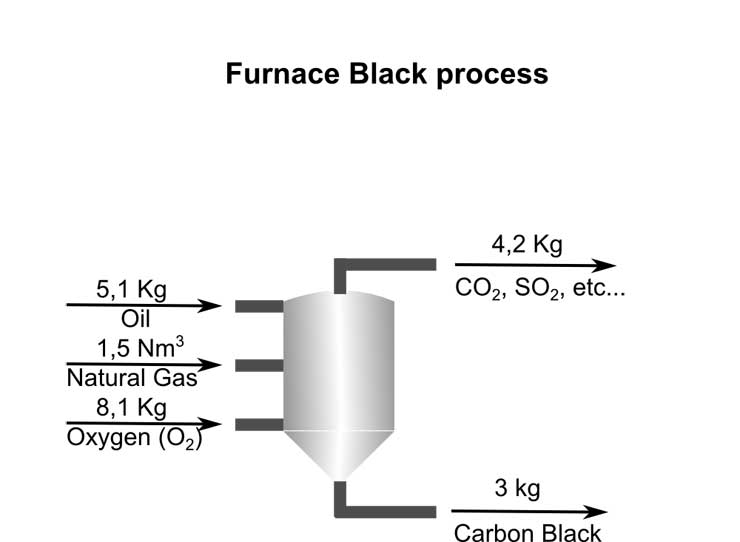
Carbon Black is currently mainly being produced by the Furnace Black process, which in addition to Carbon Black produces a variety of undesired gases including CO2, SOx and others.
For comparison: Water electrolysis, if the electrical power is produced from Natural Gas will generate around 0,4 Nm3 Carbon Dioxide per Nm3 Hydrogen produced. If the electrical power is produced from renewable sources there will be no Carbon Dioxide generation.
Plasma Assisted Decomposition – Pro & Con
Pro:
- Low emissions
- Cost effective
- Enables continued development and exploitation of oil and gas resources but in a low emission regime
- Commercially attractive by product (Carbon Black)
Con:
- Low “well to wheel” efficiency, since some of the energy of the raw material is not utilised due to the extraction and use of Carbon Black for non energy purposes.
- Possible overproduction of Carbon Black